What is Supercritical Carbon Dioxide (CO2)?
In chemistry, the state of matter is described by what is known as a phase. The phase of a substance is determined by temperature and pressure. The most common phases we experience are solids, liquids, and gases. Another phase called “supercritical” which is neither a liquid or gas but rather a mixture of the two phases. Supercritical fluids can also be thought of as an extremely dense vapor with liquid type properties.
Extraction solvents work by allowing soluble compounds to dissolve with similar polarities. Supercritical CO2 becomes an excellent extraction solvent because it allows for extraction of many non-polar compounds, such as THC and CBD, while using non-hazardous environmentally friendly green technology. Learn more about chemical polarity.
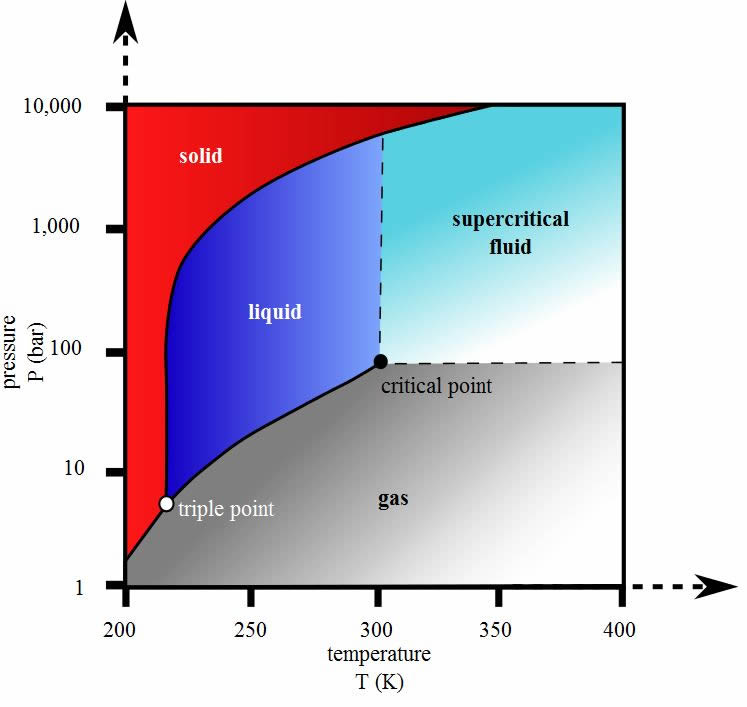
Carbon dioxide pressure-temperature phase diagram showing the triple point and critical point of carbon dioxide
Liquid carbon dioxide is a good solvent for many lipophilic organic compounds and is used to remove caffeine from coffee. Carbon dioxide has attracted attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It is used by some dry cleaners for this reason (see green chemistry). It is used in the preparation of some aerogels because of the properties of supercritical carbon dioxide.
Carbon Dioxide Phase Diagram – describes the phase of matter as dependent on the pressure and temperature of the substance. The “triple point” on the phase diagram, which all substance have, describes the temperature and pressure at which all three phases, solid liquid gas, exist simultaneously. The “critical point” on the phase diagram, which all substance have, describes the temperature and pressure at which the substance no longer can be defined as a liquid or gas and has reached a supercritical state of matter.
Difference in Extraction Methods
Supercritical fluid extraction (SFE) by CO2 is different from other types of extraction due to the required temperature and pressure needed to be in the supercritical phase. Other types of solvent extraction such as ethanol can be used at room temperature and atmospheric pressure.
Pros
- Environmentally friendly
- Non-flammable
- Renewable source of cheap solvent
- Non-toxic
- Selective extraction ability through temperature and pressure variation
- High efficiency
- Allows for pure compounds uncontaminated by solvent
Cons
- Large investment required for SFE system
- Requires employee to operate
- Some maintenance required
- Requires compressed CO2 gas cylinders
- Potential high pressure safety hazard
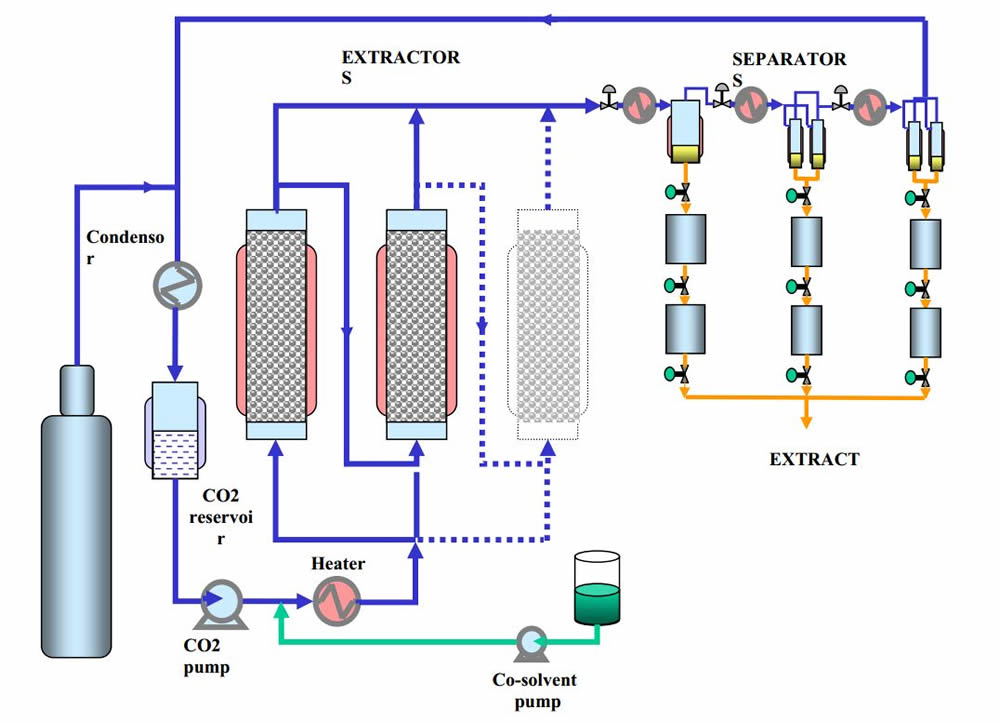
Supercritical Fluid Extraction Process Cycle
Liquid CO2 from the reservoir is circulated in the closed system loop by a pump into the heated high pressure extraction vessel where it become supercritical. The supercritical fluid extracts non-polar compounds as it moves through the extraction vessel which is then channeled into a separator/collection vessel. As the supercritical fluid drops in pressure to a sub-critical phase the extracted compounds separate and drop to the bottom of the collection vessel. The CO2 gas then moves into a heat exchanger where it is cooled and further condensed back in the CO2 liquid reservoir. This cycle is repeated over a period of time until all of the desired compounds have been extracted.
Co-Solvent Effects
Various levels and combinations of [CO2] temperature and pressure alters the “solvent power” or degree of solubility which allows the compound of interest to be selectively extracted from the material. The properties of supercritical CO2 will only allow non-polar compounds to be extracted. To modify this property, a small percentage of a polar co-solvent such as ethanol can be added to extract more polar type compounds.